- MFR: GE Healthcare
- MODEL: GEM H8031VA
Description
Additional Information
| Weight | 1 lb |
|---|---|
| Equipment Type | Diagnostic Instruments |
| Brand | GE Healthcare |
| Condition | Factory New |
| Model | Vscan Air Portable Ultrasound System |
| Warranty | 3 Year Warranty |
Brochure
Specifications
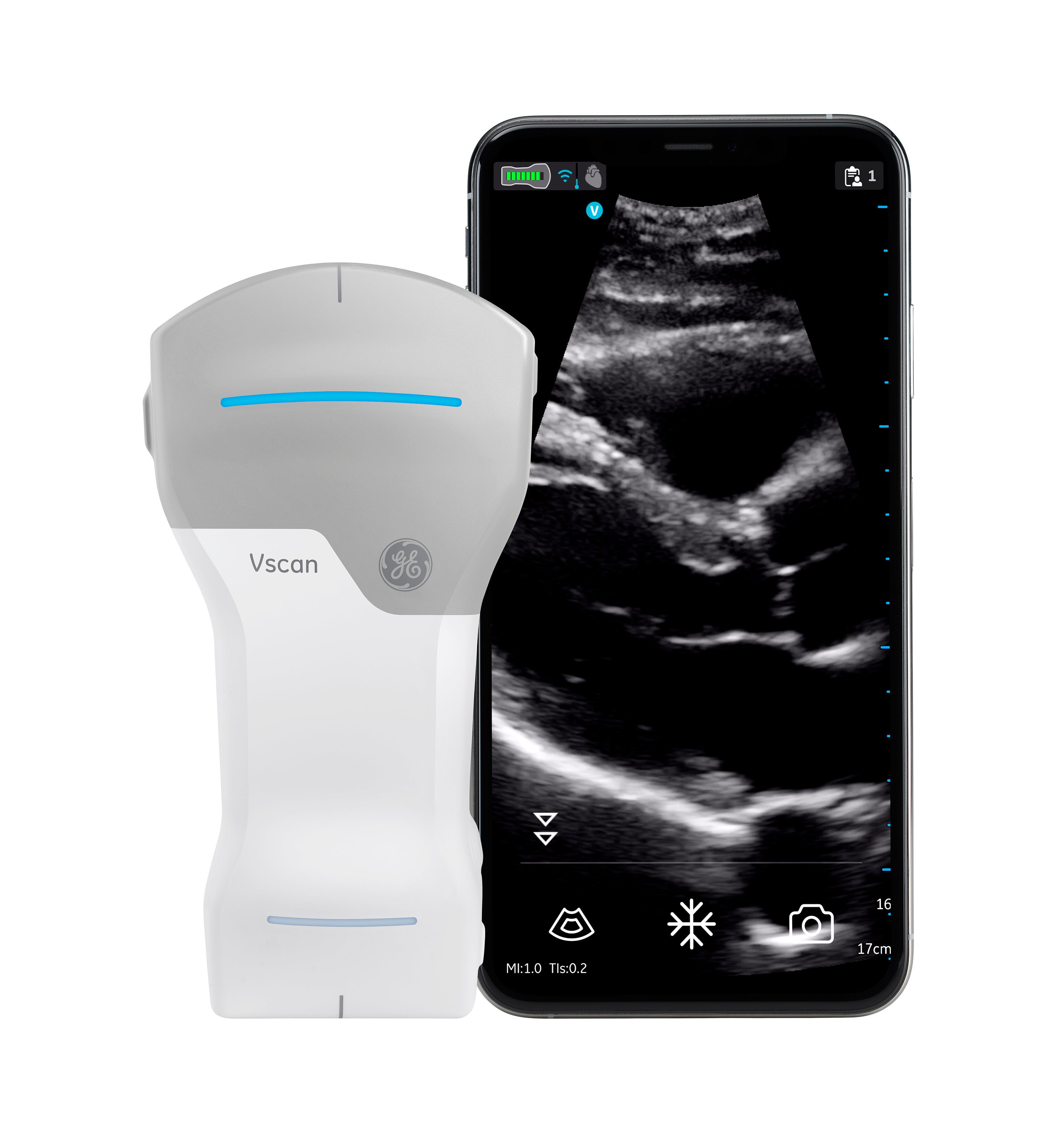
Vscan Air CL Overview
- Handheld, battery-powered ultrasound system for use by trained healthcare professionals.
- Designed for visualization, measurement, and guidance during diagnostic and interventional procedures.
- Dual-headed probe with a curved (convex) transducer on one end and a linear array transducer on the other.
- Works with the Vscan Air app on compatible Android™ and iOS® smartphones and tablets.
- Suitable for hospitals, clinics, medical offices, ambulances, and home or community care environments.
- Supports focused assessments and adjunctive use with other clinical data in adult, pediatric, and neonatal patients.
General System Characteristics
- 128 physical channel beamforming.
- Harmonic imaging to enhance signal-to-noise ratio and reduce side-lobe and reverberation artifacts for improved tissue detail.
- Approximate continuous scan time: 50 minutes per full battery charge (assuming ~80% B/W and 20% color imaging).
- Approximate recharge time from 10% to 90% battery: 75 minutes.
- Smart connect pairing to keep the probe associated with the display device while charging.
- Approximate probe dimensions: 131 × 64 × 31 mm.
- Approximate probe weight: 205 ± 3 g.
- Ingress protection rating: IP67.
- Drop-tested to MIL-STD-810G, Method 516.7, Table 516.7-7.
- Storage mode allows the probe to be stored for up to 36 months with limited battery degradation.
Curved Array Transducer (Deep Scanning)
- Typical applications: abdominal, fetal/obstetric, gynecological, urological, thoracic/lung, adult and pediatric cardiac (≥40 kg), peripheral vascular, musculoskeletal, pediatric, and interventional guidance (e.g., freehand needle or catheter placement, fluid drainage, nerve block, biopsy).
- Broad-band curved array with frequency range approx. 2–5 MHz (center ≈ 3.3 MHz).
- Number of elements: 128.
- Approximate lens footprint: 64 mm × 16 mm.
- Approximate viewing angle: 60°.
- Maximum imaging depth: up to 24 cm.
Linear Array Transducer (Shallow Scanning)
- Typical applications: peripheral/vascular, musculoskeletal (conventional and superficial), small parts, thoracic/lung, ophthalmic, pediatric, neonatal head, and interventional guidance (e.g., vascular access, fluid drainage, nerve block, biopsy).
- Broad-band linear array with frequency range approx. 3–12 MHz (center ≈ 7.7 MHz).
- Number of elements: 192.
- Approximate lens footprint: 40 mm × 7 mm.
- Maximum imaging depth: up to 8 cm.
Imaging Modes
- B-Mode – real-time two-dimensional grayscale imaging.
- Color Doppler – color overlay to visualize blood flow direction and presence.
- Pulsed Wave Doppler (PW) – spectral display of velocity and direction of blood flow for quantitative measurements.
- M-Mode – motion display of tissue structures along a single line over time.
User Interface & Workflow
- Thumb-optimized touchscreen interface with a minimal number of keys and gestures.
- App supports both portrait and landscape orientation to suit different scanning positions and ergonomics.
- Single key or gesture controls for:
- Freeze / Unfreeze
- Store image or clip
- Gain adjustment
- Depth adjustment
- Two-step process to change imaging preset for the selected transducer.
- Two-step process to review images from the current exam.
- Organ-specific presets with user-selectable default preset when opening the app.
- Measurement tools include distance, circumference, area, angle, velocity, slope, heart rate, bladder volume, and obstetric parameters (BPD, HC, AC, FL, CRL, AFI, DVP, AOP).
- Annotation options: arrow and free text.
- Transducer element check functionality.
- Optional centerline marker display.
- Optional TGC (Time Gain Compensation) with six depth-dependent gain controls.
- Swipe-in menu for easy access to configuration and management functions, including:
- Enable/disable TGC controls and preview mode.
- Enable complementary storage of binary image data.
- Settings for auto-freeze behavior and video clip duration.
- Configuration of the probe button (freeze or store).
- Downloadable user manual in supported languages.
- Diagnostics tools with log-file upload to GE HealthCare support.
- Direct links to customer support and cloud-based educational resources.
- Information on software versions for both probe and app, with the option to unregister or re-register the device.
Image Quality Technologies
- SignalMax™ signal-processing technology combines compact hardware with high transmit power for high-quality portable imaging.
- High-Definition Speckle Reduction Imaging (HD-SRI)* helps improve contrast resolution and smoothness while preserving important image details.
- *HD-SRI is offered as a non-standard/optional feature in some configurations.
Data Storage & Management
Patient Identification
- Manual entry of patient details at the start of the exam.
- Support for DICOM® Modality Worklist to standardize exam and image labeling before export.
- Ability to query Modality Worklist by patient ID.
Exam Storage on Device
- Capacity for approximately 500 exams on the mobile device (device-dependent).
- Still images stored in generic JPG format; video clips stored as MPG.
- Optional storage of binary image data for advanced analytics in collaboration with GE HealthCare.
- Exams organized as collections of images and clips, linked to patient information when entered.
- All stored exams can be retrieved on the device for review.
Data Export
- Ability to share anonymized images and video clips with other apps on the mobile device.
- Wireless export of images, clips, or complete exams (with or without patient information) in JPG or MP4 format to shared network folders.
- Wireless export of exams with patient data in DICOM format to DICOM PACS.
- Wireless export using DICOMweb™ (STOW-RS over TLS) to MyImageCloud1 or other compatible third-party cloud storage.
- Supports MPPS (Modality Performed Procedure Step).
Supported DICOM Services
- Verify
- Modality Worklist
- Store
- Storage Commitment
- Secure DICOM (TLS)
- Secure DICOMweb STOW-RS (TLS)
Data Security
Security for Data at Rest
- Vscan Air app can only be opened after the mobile device itself is authenticated (e.g., device lock/unlock).
- Additional protection via app-specific user accounts and passwords.
- Support for multiple user accounts on a shared mobile device.
- Images and patient data stored in a private app space, isolated from other mobile apps.
- Images on the device are saved without embedded patient identifiers; linkage occurs via an encrypted patient database.
- Database encryption aligned with FIPS 140-2 (AES-256-bit).
- Optional extra protection with PIN or biometric access (Face ID or fingerprint) to open patient data within the app.
- Configurable security where exam data is erased after 10 incorrect PIN attempts.
Security for Data in Transit
- Images anonymized before sharing with other mobile apps.
- Support for enterprise-level Wi-Fi security including EAP and WPA2™ (PSK).
- TLS encryption with optional mutual authentication for secure DICOM transfers.
- Configurable automatic removal of images from the device after confirmed DICOM Storage Commitment.
- MyRemoteShare1 sessions encrypted end-to-end over TLS.
- Exam and image export to MyImageCloud1 via secure DICOMweb (STOW-RS over TLS).
Secure Authentication & SSO
- Complex password support with automatic sign-out options.
- Supports integration with enterprise Identity Providers (IdP) for Single Sign-On (SSO).
- Typical prerequisites include public-facing federation servers, firewall rules for HTTPS (e.g., port 443), and reliable internet connectivity.
- Example software options include ADFS and Azure AD Connect, with requirements for encrypted data in transit via TLS/SSL.
- Supported protocols: SAML v2 (preferred), OpenID Connect (OIDC)/OAuth 2.0 (preferred), and read-only LDAPS over secure channels.
Digital Tools & AI Features
Auto Bladder Volume1
- AI-assisted bladder volume measurement designed to help reduce unnecessary catheterization.
- Automatically detects bladder boundaries and places measurement calipers.
- Clinician maintains control and can adjust measurements before the system calculates final volume.
MyRemoteShare2
- Enables live audio conversation with a remote participant while scanning.
- Shares the Vscan Air app screen; optional sharing of video from the mobile device camera.
- Remote participants join via a standard web browser on computer or mobile devices using a secure link.
- Powered by Zoom’s secure healthcare platform (not intended for primary diagnostic use for the remote viewer).
MyImageCloud
- Cloud-based storage and exam management for DICOM images exported from the Vscan Air app.
- Accessible via sign-in at myvscan.gehealthcare.com using the same credentials as the app.
- Allows review of uploaded exams and sharing with selected peers or colleagues.
MyDeviceHub
- Enterprise tool for managing a fleet of Vscan Air devices.
- Accessed by designated administrators through myvscan.gehealthcare.com.
- Supports device registration, user access control, and centralized configuration of DICOM servers.
Display Devices & Operating System Options
- GE HealthCare recommends using mobile devices that have been tested and validated for compatibility (listed in the Vscan Support Center).
Supported Operating Systems
- Android phones and tablets:
- OS version 12, 13, 14 or newer.
- 64-bit ARM CPU architecture with 64-bit kernel.
- Minimum 4 GB RAM.
- Android OpenGL ES 3.0 support.
- Compatibility with Google Play™ Store.
- iPhone devices with iOS 16, 17, 18 or newer.
- iPad devices with iPadOS 16, 17, 18 or newer.
Screen & Memory Requirements
- Screen size: approx. 5–20 inches.
- Resolution: at least 960 × 640 (or 640 × 960) pixels.
- Internal storage: at least 8 GB free space.
Connectivity Requirements
- IEEE 802.11n Wi-Fi.
- Wi-Fi Direct (Android only).
- 5 GHz band support.
- Bluetooth® Low Energy 4.0.
Security Requirements for Mobile Device
- Support for WPA2™ Wi-Fi security.
- Device-level data encryption and enabled user authentication.
Standard Configuration
- Vscan Air CL probe.
- Vscan Air app (for iOS and Android)3.
- Protective carrying case.
- Wireless charger with USB cable.
- Country-specific AC adapter4.
- Quick Start Guide.
- Electronic user manual.
Available Accessories
- Printed user manuals in additional languages.
- Extra protective carrying case.
- Additional wireless charger with USB cable.
- trophon® Wireless Ultrasound Probe Holder1.
- AC Adapter Multiplug1.
- Vscan Air Roll Stand1.
User Support & Education
Vscan Support Center
- Online hub with product information, service and clinical support resources.
- Hosts educational content such as webinars, thought-leadership pieces, and training materials.
Ultrasound Education Solutions
- Point of Care Ultrasound FocusClass by 123sonography1 – approx. five hours of video content with demos and clinical examples covering cardiac, OB, abdominal, lung, joints, carotid, eFAST, and more.
- OB/GYN Ultrasound FocusClass by 123sonography1 – online modules covering common obstetric and gynecologic indications, acquisition techniques, and case-based pathology examples.
Safety & Regulatory Conformance
- Probe classified as internally powered medical electrical equipment with type BF applied parts (per IEC 60601-16).
- Vscan Air CL probe (model Vscan Air CL) is CE-marked under MDR (2017/745/EU), RED (2014/53/EU), RoHS (2015/863/EU), and complies with WEEE (2012/19/EU).
- Vscan Air CL models C1 and G1 are CE-marked under the Medical Device Directive (93/42/EEC), RED, RoHS, and WEEE.
- Vscan Air R3 for Android and iOS are CE-marked under MDR.
- Probe certified by NRTL to CAN/CSA-C22.2 No. 60601-1 and ANSI/AAMI ES60601-1.
- Wireless charger pad certified to IEC/EN 62368-1.
Key Safety Standards (Selection)
- IEC 60601-1 – General requirements for basic safety and essential performance.
- IEC 60601-1-2 – Electromagnetic compatibility requirements and tests (Group 1, Class B per CISPR 11 / EN 55011).
- IEC 60601-2-37 – Particular requirements for ultrasonic diagnostic and monitoring equipment.
- IEC 60601-1-11 – Home healthcare environment requirements.
- IEC 60601-1-12 – Requirements for emergency medical services environments.
- EN 13718-1 – Requirements for medical devices used in air ambulances.
- EN 1789 – Requirements for medical devices used in road ambulances.
- ISO 10993-1 and related parts – Biological evaluation of medical devices.
- IEC 62304 – Medical device software life-cycle processes.
- IEC 62366-1 – Usability engineering for medical devices.
1 Some options, tools, or accessories may not be available in all countries or configurations.
2 Remote viewing quality depends on network and device; MyRemoteShare is not intended as a primary diagnostic display for remote participants.
3 The Vscan Air app is downloaded from the App Store or Google Play and becomes a medical device after registration and activation per instructions.
4 One AC adapter with an appropriate plug type (e.g., A, C, F, G, or I) is included, based on region.
Literature
- MFR: GE Healthcare
- MODEL: GEM H8031VA
Request a Quote
Shipping
Standard Ground: Most items ship free to the lower 48 states.
Freight: Oversized items ship for $495 each, secured to a pallet and delivered curbside to your address. This does not include moving the shipment inside, unpacking, or disposing of packing materials—you are responsible for these tasks.
White Glove: This includes unloading, unpacking, packing material disposal, and placement in your desired location. If you would like to inquire about white glove delivery please contact us for a quote.
Returns + Exchanges
Hassle-Free Returns: We offer hassle-free returns within 30 days of receipt. Opened brand-new items are subject to a 20% restocking fee, and customers are responsible for return shipping costs. However, if the item is misrepresented or arrives damaged, we will cover the return shipping. To initiate a return, simply contact us.
Price Match Guarantee
We’re committed to offering the best prices online! If you find a lower price from a competitor, we’ll match it—or even beat it. Exclusions apply to auction sites, third-party marketplaces like eBay, and unauthorized sellers. Contact us to request a price match!
Sales Tax Exemption
If you qualify for sales tax exemption, we’re happy to help! Contact us with your exemption certificate, and our team will assist you in setting up a tax-free purchase.
Estimated Delivery
Delivery estimates are calculated by adding the estimated handling and shipping time.
For further information, please refer to our Shipping Policy.
Factory New Items
Factory New items are brand new and come with a manufacturer's warranty. They may be drop shipped directly from the manufacturer's factory.
Warranty Policy
Warranty coverage varies depending on the product, its condition (Factory New, Factory Refurbished, and Certified Refurbished), and the manufacturer.
- Factory New Items are covered by the manufacturer's warranty. All warranty claims must be processed through the original manufacturer.
- Refurbished Items are also covered by Quince Medical & Surgical.
You may also like
- Opens in a new window.